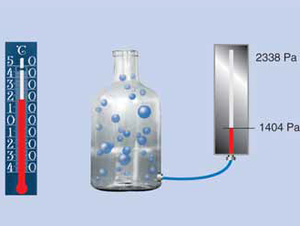
Diffusion of vapour
Water is one of the most important substances on the planet. Without water there is no life. And so water is found virtually everywhere – in the ground, in the air, in building materials, everywhere. It can change its form. We distinguish between three states:
- Solid (ice): The freezing point of water is 0 °C, and below this temperature, water becomes ice.
- Liquid (water): At temperatures above 0 °C water is found in its "normal" form, as we encounter it daily.
- Gaseous (water vapour): Water vapour is invisible. It is contained in varying amounts in the air surrounding us, and in fact at any temperature, even far below 0 °C.
Nice to know!
We have to deal with water in all three of its states when it comes to managing buildings. The problem of diffusion of vapour mainly involves gaseous water. The ability of water to change its form at varying temperatures poses problems for the planning and execution of construction works.We have to deal with water in all three of its states when it comes to managing buildings. The problem of diffusion of vapour mainly involves gaseous water. The ability of water to change its form at varying temperatures poses problems for the planning and execution of construction works.
The air humidity
Gas mixture
This mixture of different gases, our air, has a certain atmospheric pressure, which varies depending on the weather and altitude. The amount of water vapour in air is referred to as air humidity content.
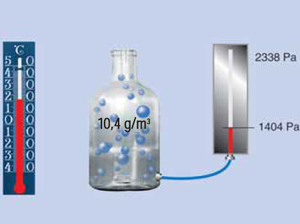
Absolute air humidity
The absolute air humidity ϱD (pronounced as Rho D) refers to the actual water vapour content in the air. It is expressed in g/m 3.
Water vapour diffusion
According to a fundamental physical law, the natural tendency of gases of differing concentrations is to mix. Thus the water vapour molecules will try to move from the side of higher concentration to the side of lower concentration, until a balance is reached. We refer to this migratory movement as water vapour diffusion.
The air humidity
The air mainly consists of oxygen and nitrogen, in addition to some other gases. In addition, it can hold a certain amount of water vapour. The capacity to hold water vapour depends on the temperature of the air, and it increases as the air temperature increases. Warm air can therefore hold more water vapour than cold air.
Physical parameters of diffusion of air humidity
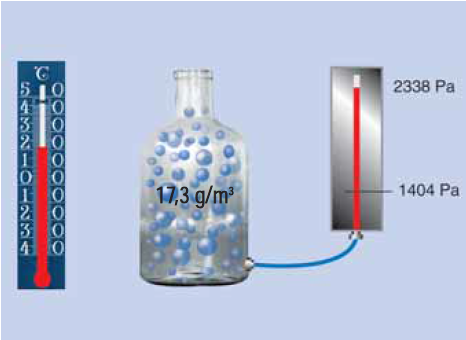
Maximum air humidity
Depending on the temperature, air can absorb only a certain maximum amount of water. This amount is referred to as maximum air humidity in g/m3. The maximum air humidity corresponds to the saturation pressure (ps) of the water vapour at the respective temperature.
Water vapour in residential buildings
Water vapour and partial pressure
In inhabited buildings, a large quantity of water vapour is generated, which may cause the partial pressure p to rise sharply. However, partial pressures do not produce overpressure of the air and are therefore not noticeable.
- Occupants: A person's breath and perspiration produce 1 to 2 l of water vapour per day. Therefore, in a household with 4 persons (2 adults + 2 children) approximately 5 l of water vapour is given off.
- Cooking: Cooking in this 4-person household produces up to 2 l water vapour daily.
- Bathing, washing, plants, etc.: Bathing, showering, washing, house plants, etc. additionally give off 3 l water vapour every day in our 4-person household. Thus a total of around 10 l water vapour per day is emitted in this household.
Nice to know!
In a 4 1/2 room house with 300 m3 of space from which water vapour cannot escape, the ambient air at 20 °C could absorb up to 300 x 17.31 g/m3 = approximately 5 l of water vapour, and then it would be saturated! The other 5 l would deposit in the form of condensation on windows, walls, furniture, etc. The house would thus become a true "dripstone cave"!