Water vapour diffusion
Water vapour molecules

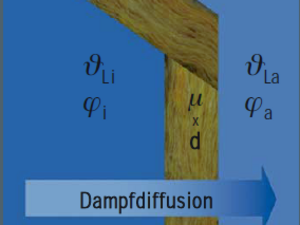
A water vapour molecule has the size of only one ten-millionth of a millimetre (0.0000001 mm); therefore it is totally invisible to us. This also explains why substances that are absolutely impervious to water are relatively easily penetrated by water vapour. If a vessel were to be made of porous building materials instead of glass, then the pressure compensation would take place in the shortest possible time. The water vapour molecules would pass through the walls of the vessel. This process is what we call water vapour diffusion. Depending on the material of the walls, they offer more or less resistance to water vapour diffusion. The parameter for this is the diffusion resistance factor μ (pronounced as 'myu'). It indicates how high the diffusion resistance of the corresponding material is with respect to that of air of equal layer thickness.
Nice to know!
- Air has a diffusion resistance factor of μ = 1
- Mineral wool has a diffusion resistance factor of μ = 1.5
- A vapour check, e.g. Ampatex DB 90 (thermoset endless fibres with polypropylene fill) has a vapour diffusion resistance factor of μ = 68,000
- A vapour seal, e.g. Sisalex 514 (kraft paper + paraffin + glass fibre fabric + aluminium) has a vapour diffusion resistance factor μ = 6,900,000
Diffusion resistance
Diffusion resistance factor
The diffusion resistance factor μ is a constant, and is not dependent on layer thickness. It is not particularly relevant for the construction site, because each material has a different thickness. Therefore, we compare the diffusion resistance of a material usually used in construction (e.g. 20 cm concrete) with the resistance of an air layer of 1 meter thickness. We call this value diffusion equivalent air layer thickness sD, which we indicate in meters (m).
Water vapour diffusion
Water vapour condensation
We know that water vapour migrates. This is also the case with exactly the same temperatures inside and outside the building, because there is a difference in relative air humidity φ and thus a partial pressure difference Δp (pronounced as delta P) exists.
This vapour diffusion is harmless, so long as a significantly lower temperature does not exist on the side with less pressure. If this is the case, it may be critical. The temperature difference is called Δϑ (pronounced as delta theta).
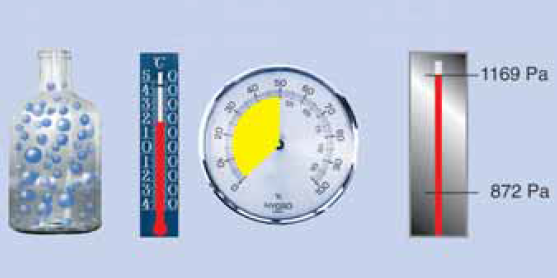
First of all, it is of utmost importance to know that the maximum amount of water vapour that the air can absorb depends particularly on the air temperature ϑ (pronounced as theta).
- At 30 °C, the air can absorb max. 30.40 g/m3 of water vapour. (ps = 4,241 Pa).
- At 20 °C, the air can only absorb max. 17.31 g/m3 of water vapour. (ps = 2,337 Pa).
- At 10 °C, the air can only absorb max. 9.41 g/m3 of water vapour. (ps = 1,227 Pa).
- At 0 °C, the air can only absorb max. 4.85 g/m3 of water vapour. (ps = 611 Pa).
- At -10 °C, the air can only absorb max. 2.14 g/m3 of water vapour. (ps = 260 Pa).
- At -20 °C, the air can only absorb max. 0.88 g/m3 of water vapour. (ps = 103 Pa)

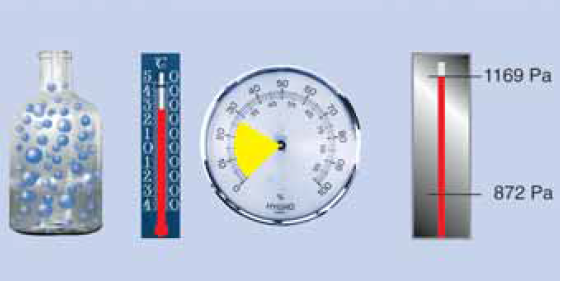
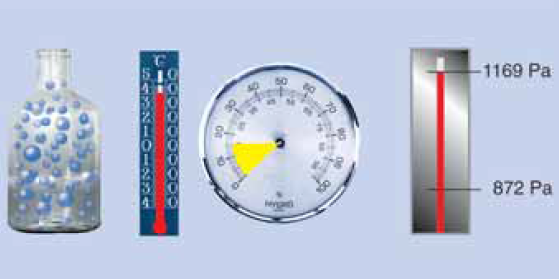
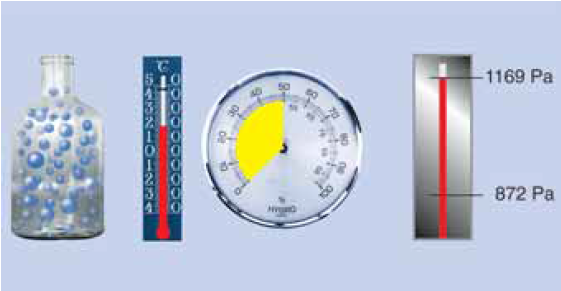
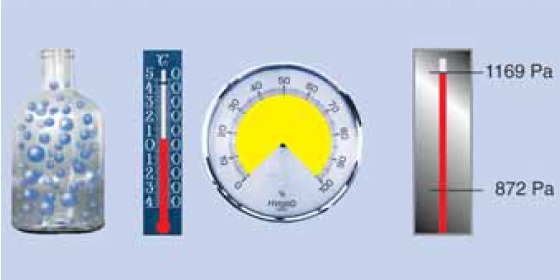
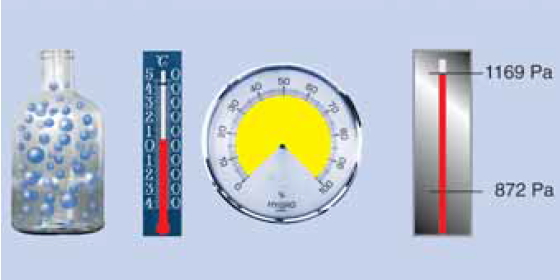
Water vapour condensation
Now further cooling inevitably leads to condensation. At 5 °C the air can still have only a maximum of 6.8 g/m3, the remaining 1.85 g of water vapour condenses to water! The saturation pressure ps is now only 872 Pa!
This series can also be continued, but the result will be much more disastrous than in the case of continued heating. At -20 °C, already here 7.8 g of water per m3 of air would accumulate!
Water vapour condensation

Surface condensation during vapour production
If a lot of vapour is produced, for example, while taking a shower, φ rises typically to 80% at 22 °C. The dew point temperature ϑT for this climate is 18.4 °C. If the window pane has a surface temperature of less than 18.4 °C now, condensation water deposits on it!

Condensation zone in structural components
If the diffusion of vapour takes place at a large temperature gradient, then a pronounced condensation zone can occur in strongly vapour-permeable single-layer building materials. It is located in the part of the layer which is colder than the dew point temperature ϑT.
Water vapour in buildings
Exactly the same happens when, on its way through the construction, the water vapour which is continually produced in the building (see example P. 11) passes over components or air layers whose temperature ϑ (theta) is below the dew point temperature ϑT (theta T) relevant for the indoor climate.